
HARMONi-3 Phase 3 Clinical Trial
1L Metastatic NSCLC
NCT05899608: Click to view on ClinicalTrials.gov
A randomized, double-blind, multi-regional phase 3 Study of ivonescimab combined with chemotherapy versus pembrolizumab combined with chemotherapy for the first-line treatment of metastatic NSCLC.
HARMONi-3 Study Schema
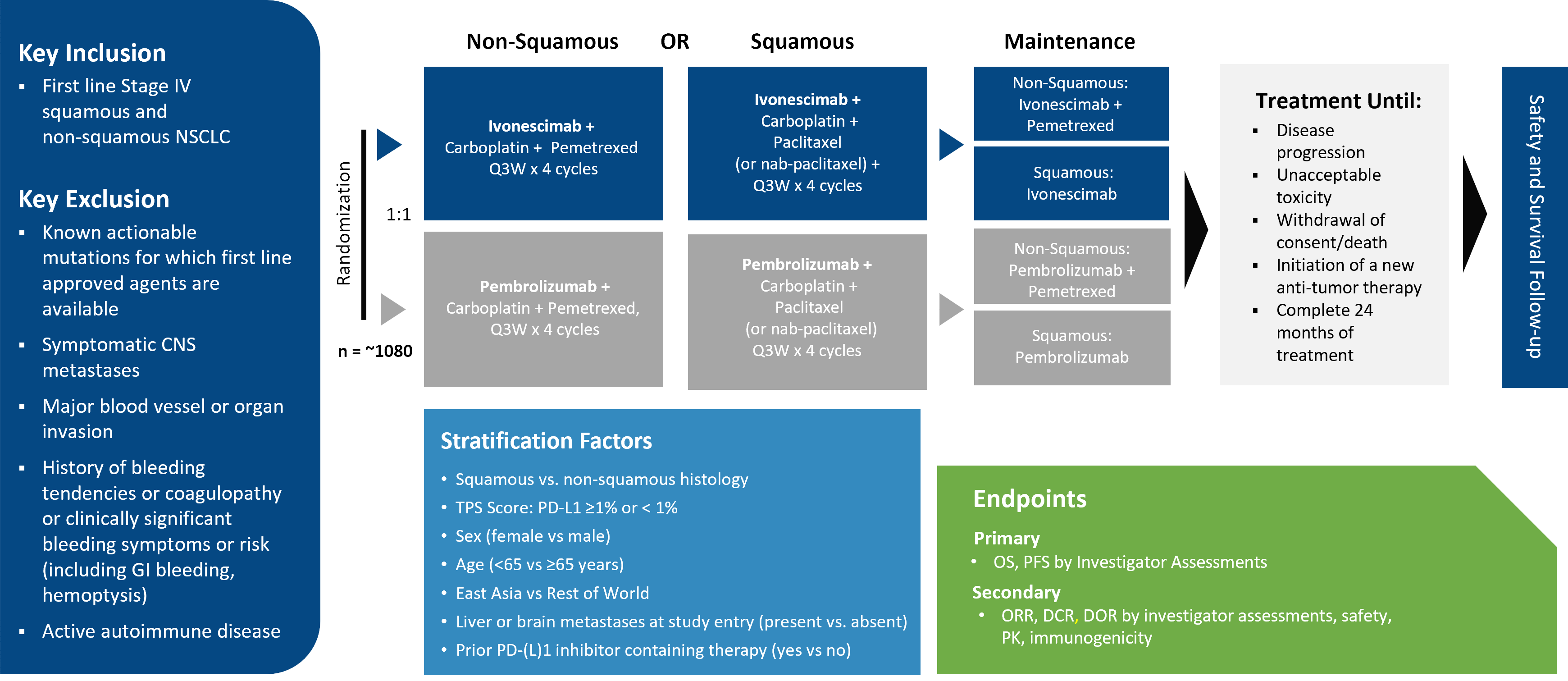
Key Eligibility Criteria
- Metastatic (Stage IV) NSCLC
- Histologically or cytologically confirmed squamous or non-squamous NSCLC
- Patients must have Tumor Proportion Score (TPS) with PD-L1 expression prior to randomization
- No prior systemic treatment for metastatic NSCLC. No histologic or cytopathologic evidence of the presence of small cell lung carcinoma, or non-squamous NSCLC histology
- No known actionable genomic alterations in EGFR, ALK, ROS1 or BRAF V600E or genes for which first-line approved therapies are available
- No Radiographic evidence of major blood vessel encasement with narrowing of the vessel or intratumor lung cavitation or necrosis that the investigator determines will pose a significantly increased risk of bleeding.
- No symptomatic CNS metastases or CNS metastasis ≥1.5 cm
- No history of bleeding tendencies or coagulopathy and/or clinically significant bleeding symptoms or risk within 4 weeks (including GI bleeding, hemoptysis)
Ivonescimab is an investigational therapy that is not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA).
For additional information on the HARMONi-3 Clinical Trial, please contact medinfo@smmttx.com
