
HARMONi-7 Phase 3 Clinical Trial
1L Metastatic NSCLC
NCT06767514: Click to view on ClinicalTrials.gov
A Randomized, Double-blinded, Multiregional Phase 3 Study of Ivonescimab Versus Pembrolizumab for the First-line Treatment of Metastatic Non-small Cell Lung Cancer in Patients Whose Tumors Demonstrate High PD-L1 Expression.
HARMONi-7 Study Design
Monotherapy Ivonescimab vs. Pembrolizumab
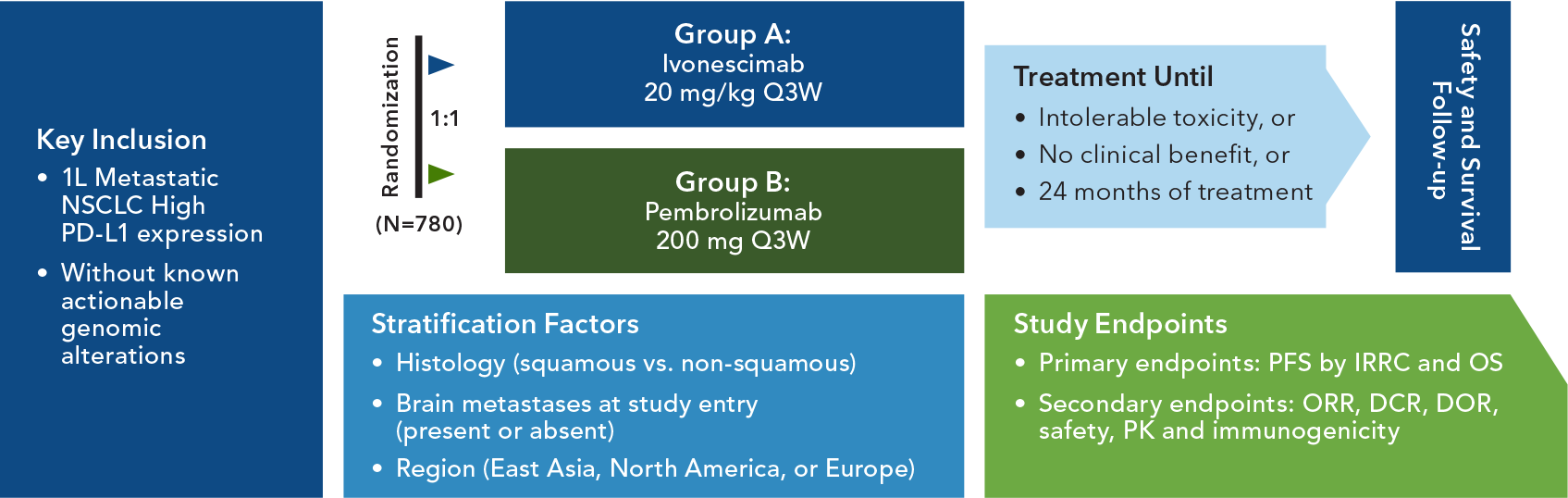
Key Eligibility Criteria
- Metastatic (Stage IV) NSCLC, PD-1 ≥50%
- ECOG 0 or 1
- Histologically or cytologically confirmed squamous or non-squamous NSCLC. No histologic or cytopathologic evidence of the presence of small cell lung carcinoma
- Patients’ tumor must have high PD-L1 expression
- No prior systemic treatment for metastatic NSCLC
- No known actionable genomic alterations in EGFR, ALK, ROS1 or BRAF V600E for which first-line approved therapies are available
- No radiologically documented evidence of major blood vessel invasion, or tumor invading organs, or major blood vessel encasement with narrowing of the vessel or intratumor lung cavitation or necrosis that the investigator determines will pose a significantly increased risk of bleeding
- No symptomatic CNS metastases or CNS metastases with hemorrhagic features or CNS metastases ≥1.5 cm
- No history of bleeding tendencies or coagulopathy and/or clinically significant bleeding symptoms or risk within 4 weeks
Ivonescimab is an investigational therapy that is not presently approved by any regulatory authority
other than China’s National Medical Products Administration (NMPA).
Abbreviations: ALK=anaplastic lymphoma kinase; CNS=central nervous system; DCR=disease control rate; DOR=duration of response; ECOG=eastern cooperative oncology group; EGFR=epidermal growth factor receptor; IRRC=independent radiologic review committee; NSCLC=non-small cell lung cancer; ORR=overall response rate; OS=overall survival; PD-1=programmed cell death protein 1; PD-L1=programmed death-ligand 1; PFS=progression-free survival; PK=pharmacokinetics; Q3W=every 3 weeks; VEGF=vascular endothelial growth factor.
For additional information on the HARMONi-7 Clinical Trial, please contact medinfo@smmttx.com
